Link: https://www.sciencedirect.com/science/article/pii/S246882311930032X
Title: Molecular mechanism of heterogeneous supramolecular catalysis of metal-free cucurbituril solid for epoxide alcoholysis
Authors: Lina Xu, Guoyong Fang*, Yanghong Yu, Yuefan Ma, Zihang Ye, Zhenyu Li*
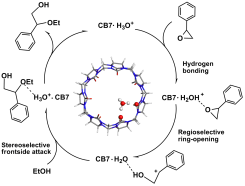
Abstract: Heterogeneous and supramolecular catalysis are fundamental processes in chemistry. To understand the synergistic role between heterogeneous and supramolecular catalysis, the catalytic mechanism of cucurbituril solid for epoxide alcoholysis was investigated by performing density functional theory (DFT) calculations. The results reveal that styrene oxide (StyOx) ethanolysis has an inherent regioselectivity, which results from different groups linked to the epoxy group. The hydronium ion can catalyze the ring-opening of StyOx ethanolysis and lead to the formation of a planar carbenium ion. StyOx can also be hydrolyzed via the homogenous catalysis of acid to produce 1, 2-diol. Cucurbituril solid can catalyze epoxide alcoholysis because of its acidic property. Its unique cavity can lead to a favorable frontside-attack of the alcohol on the carbenium ion. The product from the heterogeneous catalysis of cucurbituril solid is pure β-alkoxy alcohol. The results are important to understand heterogeneous and supramolecular catalysis and the design of new and effective supramolecular catalysts.
TOC: